Dual-Payload ADCs: A New Chapter in Cancer Therapy
Published on.
2025-10-16 17:06
Antibody-drug conjugates (ADCs) have become one of the fastest-growing “stars” in cancer therapy by virtue of their unique mechanism of action and impressive clinical efficacy. Yet, as their use has widened, the technology’s weak spots have surfaced: resistance and toxicity are now looming larger. This raises an obvious question: if ADCs are essentially “upgraded” chemotherapy, can they—like traditional chemotherapy—synergize with combination partners to reduce toxicity, overcome resistance and push efficacy even higher?
Studies have shown that Precision-co-delivery of two synergistic payloads at a precisely controlled ratio directly into cancer cells does indeed enhance therapeutic efficacy. Combining two payloads with distinct mechanisms of action also substantially reduces the incidence of drug resistance. Exploiting such complementarity is therefore emerging as a central pillar of next-generation ADC design and a key arena for industrial differentiation.
But two bioactive molecules significantly differ from each other in mechanism of action, biological activity, PK property, minimum effective dose and toxic dose. Simply conjugating payload to antibody at a fixed 1:1 ratio is unlikely to yield an ADC with adequate efficacy and safety, compromising its druggability. Choosing appropriate payloads with the right ratio is pivotal for the successful development of dual-payload ADCs.
The strategy of combining payloads determines the complexity and feasibility of the synthetic process. There are two general strategies of constructing dual-payload ADCs. The first one is to generate the ADC at two distinct classes of sites—e.g., lysines and cysteines—using two different chemistries, with one or more payloads introduced at one class of sites in each step. The second one introduces both payloads at the same class of sites—e.g., cysteines generated by reduction—either as a pre-assembled linker–payload conjugated to the antibody or by stepwise attachment after antibody conjugation (first the linker, then the payloads). Whichever option is chosen, a branched linker is required to connect both payloads simultaneously, and the final payload ratio is determined by the selected strategy.
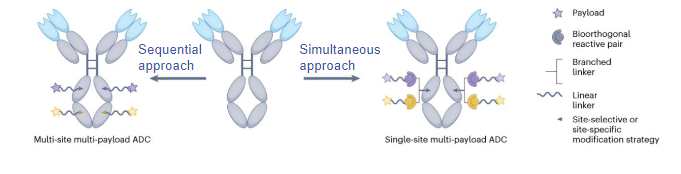
Nevertheless, dual-payload ADC construction faces several technical hurdles. First, the stoichiometry of the two payloads is dictated by the conjugation strategy and is typically locked at 1:1 or 1:2. In addition, the higher overall drug-to-antibody ratio (DAR) renders the resulting conjugate markedly more hydrophobic, inevitably compromising developability. Achieving a synergistic benefit while preserving drug-like properties therefore remains the critical challenge that dual-payload ADCs must overcome.
With its highly hydrophilic and modular ADC technology platform, PrimeLink Bio offers a robust solution for dual-payload development. The proprietary linker chemistry enables precise, stoichiometric control of two distinct payloads within a single linker–payload construct synthesized chemically, ensuring perfect compatibility between distinct payloads. Besides, PrimeLink Bio’s site-specific glyco-conjugation platform integrates seamlessly with conventional cysteine conjugation technology, affording flexible, diversified pairing strategies for dual-payload ADCs. Consequently, the company’s platform enables one-step, dual-payload conjugation with a stable and readily controlled process. Moreover, the linker’s inherent high hydrophilicity offsets the increased hydrophobicity caused by increased toxins, safeguarding the developability of dual-payload ADCs. Within the company’s programs, the incorporation ratio of the two payloads can be tuned precisely from 1:1 to 1:5, and an overall DAR of 8:8 for each payload can be achieved while the ADC molecule remains highly hydrophilic.
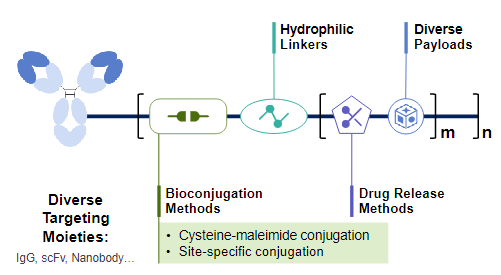

For payload selection, PrimeLink Bio follows two rules. First, give priority to payloads whose mechanisms are clearly synergistic, ensuring that the two agents cooperate within tumor cells to deliver 1 + 1 > 2 efficacy while offering a viable route to overcome drug resistance. Second, select only those payloads for which solid evidence shows that their toxicities do not accumulate in parallel, thereby maximizing therapeutic benefit without introducing additional side effects.
As a new frontier in antibody–drug conjugate research, dual-payload ADCs are dawning as a beacon of hope for oncology, marrying unique design elegance with huge therapeutic potential. Leveraging its proprietary ADC platform and deep insights into payload selection, PrimeLink Bio is advancing a pipeline of dual-payload ADCs and anticipates a stream of positive data in the near term.
About PrimeLink Bio
Founded in July 2021, PrimeLink Bio is an innovative biopharmaceutical company dedicated to the development of next-generation antibody-drug conjugates (ADCs). Guided by the principle of “tailoring optimal ADC structures for specific targets,” the company has developed a flexible, modular platform that enables novel strategies to overcome the safety liabilities and resistance mechanisms limiting current ADC therapies. Leveraging a highly hydrophilic ADC platform with outstanding compatibility and flexibility, PrimeLink Bio has built a pipeline spanning single-target ADCs and novel modalities such as bispecific ADCs and dual-payload ADCs. Three programs have already reached the PCC stage. The core pipeline’s lead candidate has shown superior efficacy and safety with best-in-class potential in preclinical studies, and its IND application was submitted in Q3 2025. With its differentiated platform and robust pipeline, PrimeLink Bio has consistently attracted top-tier investors and leading pharmaceutical companies, securing 150 million RMB in funding to date. The company will continue to advance its platform and accelerate pipeline development to bring life-changing benefits to cancer patients worldwide.